Bond Order Calculator
Bond Order Calculator is an online tool that calculates a molecule's bond order by taking a number of bonding and antibonding electrons. Use this handy tool to speed up your computations and conveniently compute bond orders.
Valence Bond Theory | Bond Order Definition
The bond order determines a molecule's or ion's stability. The stronger the connection and the higher the bond energy, the higher the bond order. There are many definitions of bond order. The bond order is the number of bound electron pairs between two atoms, according to the valence bond theory.
The bond order is defined as half of the difference between the number of bonding electrons and the number of antibonding electrons in molecular orbital theory. Let's look at some bond order examples utilising the valence bond theory.
Because it is a double bond, the bond order of the O2 oxygen molecule is 2.
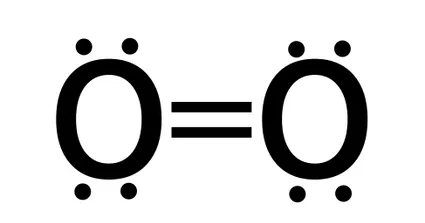
The bond order of NO3 is as follows: nitrate contains four bonds and three bond groups.
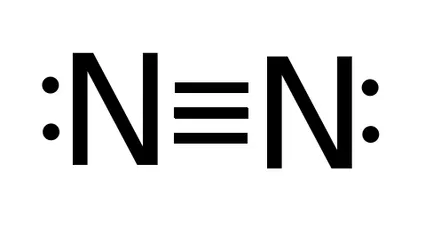
Bond order = number of bonds/number of groups = 4/3 = 1.33
FAQ’s on Bond Order Calculator
1. What exactly is a Bond Order?
The number of chemical bonds between two atoms is indicated by the bond order. The bond order of diatomic nitrogen N≡N, for example, is three, as is the bond order of carbon atoms in H≡C-H. The bond order expresses the bond's stability. The molecular orbital makes the concept of a chemical bond's bond order simple to comprehend. It measures the strength of atom-to-atom covalent connections.
2. What is the formula for bond order?
The following is the bond order of a chemical bond formula: Bond order = 1/2(Bonding Electrons - Anti Bonding Electrons)
3. What is the N2 bond order?
The bond order is three because the nitrogen molecule possesses a triple bond.
4. What is the O2 bond order?
The bond order is two because the oxygen molecule possesses a double bond.
Molecular Orbital Theory | Bond Order Formula
The bond order can be computed using the function that describes the state of an electron in a particle in molecular orbital theory. Orbitals are the names given to these functions. The orbitals show where electrons in the electron cloud are located. The following is the bond order formula Bond Order = (Number of bonding electrons - Number of antibonding electrons)/2
How to Calculate Bond Order?
Calculate the bond order of the chemical bond between antibonding and bonding electrons by following the specified steps.
- Step 1: Check the number of bonding and antibonding electrons in the system.
- Step 2: Subtract the antibonding electrons from the bonding electrons to get the bonding electrons.
- Step 3: Divide the difference by two to get the bonding order.
