Electronic Configuration Calculator
Any element's electron configuration can be determined using an online electron configuration calculator. Each element's abbreviated configuration and atomic number are displayed in this electron calculator. Continue reading to learn about abbreviated electron configuration, shells, subshells, and how to find an atom's or element's electron configuration.
What is Electron Configuration?
The electron configuration of an atom or molecule represents the distribution of electrons in distinct atomic or molecular orbitals in quantum chemistry and atomic physics. Every electron is also described as freely travelling in an orbital in an average field generated by other orbitals. For example, Phosphorus (P) has an electron configuration of 1s^2 2s^2 2p^6 3s^2 3p^3.
Physicists and chemists usually refer to the electronic configurations of molecules and atoms using standard nomenclature. The usual nomenclature for atoms consist of a succession of atomic subshell labels (for example, 1s, 2s, 2p, 3s, 3p for phosphorus), with the number of electrons assigned to each subshell used as a superscript. For example, Hydrogen has only one electron in the first shell's s orbital, hence its electron configuration notation is 1s^1. Lithium's electron configuration is 1s^2 and 2s^1, with two electrons in the 1s subshell and one electron in the 2s subshell (higher energy).
Electron Configuration Chart
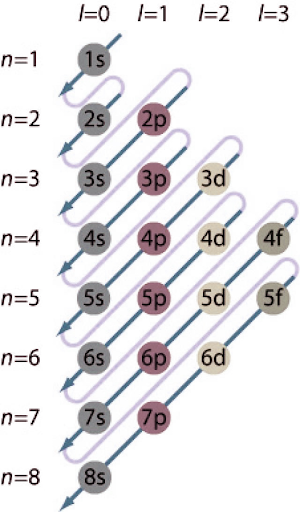
How to Calculate Electron Configuration?
A simplified method of calculating electron configuration is also shown in our free-electron configuration calculator. To write basic electron configurations by hand, follow the procedures below
- Step 1: In the beginning, check the element in the periodic table that you require. For example, Calcium is element 20.
- Step 2: In the periodic table, check the atomic number of the first noble gas.
- Step 3: To begin its electronic configuration, place the noble gas symbol in brackets. Put the noble gas's atomic number below this symbol to find out how many electrons it has.
- Step 4: Then, until you achieve the necessary amount of electrons, keep writing the electron configuration of a certain element.
Shells and Subshells
Electron shells and subshells are a collection of possible states that the electron can occupy that have the same primary quantum number n (the number before the letter on the orbital). The nth electron shell of an atom can hold 2n^2 electrons, whereas the first shell can hold 2 electrons, the second shell can hold 8 electrons, and so on.
A subshell is a collection of states determined by the shell's total azimuth quantum number "l." The ℓ value ranges from 0 to n-1. The orbits s, p, d, and f are represented by the values of ℓ = 0, 1, 2, and 3.
How to use Electronic Configuration Calculator?
To get the Electron Configuration Calculator's output, follow the steps below.
- Step 1: Fill in the required values or functions in the input field.
- Step 2: Press the "Submit or Solve" button to get the output.
- Step 3: That's all there is to it. The Final Output of your Input will now be displayed on your screen.
FAQs on Electronic Configuration Calculator
1. What is Electron Configuration?
The plan of electrons in energy levels surrounding a nuclear core is known as electronic design or electronic building. … The K–Q shells are partitioned into a lot of orbitals (see orbital) in a more detailed quantum-mechanical model, each of which can be involved by close to a pair of electrons.
2. What are the most important electron configuration rules?
The Pauli-exclusion Principle, the Aufbau Principle, and Hund's Rule are three key laws governing electron configuration.
3. How can you find out how many valence electrons there are?
Determine the number of valence electrons by using the group numbers. The number of valence electrons in an atom of a non-transition metal can be calculated using its Group number. The number of valence electrons in an atom of these elements is represented by the one place of the group number.